Jul 23, 2024 | Uncategorized
In order for the sterilization procedure to be correct, the following points should be taken into account: The chamber should be in a perfect state of cleanliness. Burden sharing should permit the free circulation of the sterilizing agent in the chamber. Each package...

Feb 27, 2024 | Scientific content
Liquid chemical method is the most frequently utilized method in our hospital system and multiple germicidal agents exist in liquid form. This method requires many controls during execution. Since it is a method that is carried out for the most part manually, all...
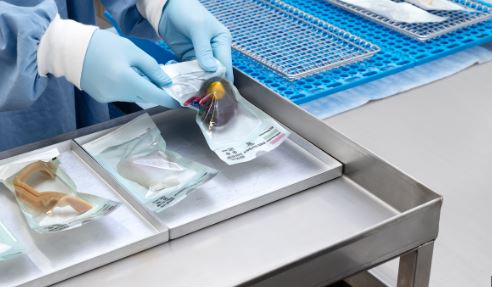
Oct 15, 2023 | Uncategorized
The manual preparation of the following models is recognized worldwide for the packaging of medical use products in the SP: Envelope type: This type is for small, rounded and light elements. The opening is made on the operator’s hand. Rectangular type: This type is...

Feb 21, 2023 | Uncategorized
Employer Responsibilities A. Employers must launder workers’ personal protective garments or uniforms that are contaminated with blood or other potentially infectious materials. Laundry Facilities and Equipment A. . Maintain the receiving area for contaminated...

Feb 7, 2023 | Scientific content, Scientific content, Scientific content, Scientific content
Before 1970, U.S. hospitals conducted regularly scheduled culturing of the air and environmental surfaces (e.g., floors, walls, and table tops). By 1970, CDC and the American Hospital Association (AHA) were advocating the discontinuation of routine environmental...





