Jul 23, 2024 | Scientific content
In order for the sterilization procedure to be correct, the following points should be taken into account: The chamber should be in a perfect state of cleanliness. Burden sharing should permit the free circulation of the sterilizing agent in the chamber. Each package...
Jul 18, 2024 | Uncategorized
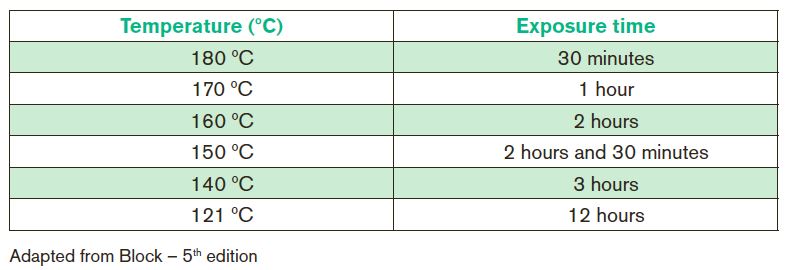
Institutional procedure manuals should establish working conditions according to the load, volume, weight and thermal resistance of the material. It is indispensable to respect the parameters obtained during the validation of the procedure. Temperature: the...

Jun 18, 2024 | Uncategorized
It is important to always take into account that the microbicidal action of heat is conditioned by the presence of organic matter or dirt on the materials. This applies, for example, to oil or fat for cases in which the microorganisms are protected from heat-based...
May 23, 2024 | Uncategorized
The emergence of HIV raised awareness concerning all of the pathogenic microorganisms that are transmitted through blood. However, national and international recommendations related to the elimination of these germs on the surfaces of the environment do not seem to be...
May 19, 2024 | Scientific content, Scientific content, Scientific content, Scientific content
Semi-critical biomedical elements contaminated with the blood of patients with HBV or HIV or with the respiratory secretions of patients with tuberculosis should receive high-level disinfection since experimental studies have demonstrated the inactivation of these...



